0 Preface
As a special commodity, if it is mismanaged and misused, it will pose a serious threat to people's health and life safety [1]. At present, there are two main problems in the circulation of drugs: the efficiency of the supply chain and the problem of counterfeiting of drugs.
Under the premise of ensuring the safety of patients, drug safety management needs to ensure the safe operation of the drug from the original manufacturing, sales, drug distribution, and finally the patient medication. This article proposes the use of the Internet of Things technology to effectively track and identify drugs from the beginning of the manufacturer to the end user (patients), to achieve drug monitoring in all aspects of the supply chain, so that patients' medication safety Get the most protection.
1 system architecture design
The drug traceability management system based on the Internet of Things makes full use of the network infrastructure to integrate the information resources of the drug supply chain collected and stored by the Internet and the Internet of Things. The system operates in the framework of security mechanisms and standards, forming a unified whole, from data collection. Three levels of data services and data applications ensure the rationality and efficiency of the system architecture.
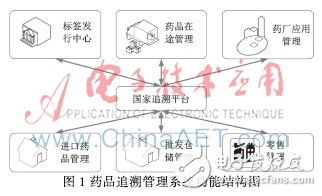
The system function structure diagram is shown in Figure 1. It includes the following functional units: national traceability platform, label distribution center, pharmaceutical application subsystem, imported drug management subsystem, wholesale warehouse management subsystem, drug in-transit management, retail management. system.
The national traceability platform implements the data center interaction and data sharing tasks. As the core module of the system, other modules interact with the platform for drug circulation data.
The label issuing center regularly collects the requirements of each pharmaceutical factory label; issues the order to the label manufacturer according to the demand of the pharmaceutical label; and performs the label issuing operation according to the label demand of the pharmaceutical company feedback.
The pharmaceutical company application subsystem is distributed in each enterprise in the country that has the qualification for the production of pharmaceutical products. Combined with the existing system, it completes the collection and reporting of the production information of the pharmaceutical products and the storage information.
The imported drug management subsystem is deployed in the drug product importer information center and warehouse, and through the coordination with the national supervision center, completes the functions of approval for the import of pharmaceutical products, collection and reporting of warehousing and logistics information.
The wholesale warehousing management subsystem is deployed in every wholesale warehouse in the country, as an intermediate link in the distribution, to complete the warehousing and distribution of pharmaceutical products.
The drug in-transit management subsystem uses GPS technology to achieve tracking and positioning and transportation information collection during drug delivery. For drugs with high transportation environment requirements, cold chain logistics vehicles are required for on-site tracking and transportation temperature monitoring.
The retail management subsystem uses the dealer's existing cash register system and the nation's comprehensive communication network, and the product sales information is directly aggregated to the national regulatory center.
The system has perfect safety mechanism and standard specifications, and cooperates with the corresponding management system to provide stable operation guarantee for the construction of drug traceability management system.
2 National retrospective platform construction
The national traceability platform provides a unified data storage, exchange and inquiry platform for drug traceability management, realizes unified management of drug circulation information, and ensures data consistency and authority.
2.1 Data Storage
2.1.1 Basic database
The basic database is used to store the business database and basic data information. The business database includes the business database of the production, wholesale, retail, and supervision of the drug. The basic information database includes the drug production information database, the drug wholesale information database, and the imported drug information database. Drug retail information database, drug regulatory information database, etc.
2.1.2 Data Warehouse
The data warehouse provides a data base for rapid analysis, query and statistics. Based on the basic support data, it forms a drug analysis topic through classification and aggregation, and supports the analysis and inquiry operation of the drug circulation data by the supervision center.
2.1.3 Supporting the database
The supporting database is used to support dedicated databases for various applications, including meta-databases, exchange databases, and so on. The metabase stores data information that interprets the data; the exchange database stores information about the application of the data exchange platform.
2.2 Data Sharing Exchange Service
During the construction of the national traceability platform, it is necessary to collect drug circulation data from drug manufacturers, drug wholesalers, retailers, importers, drug regulatory authorities, etc. There are a large number of data exchange needs, in order to complete these data exchange tasks, A unified data sharing exchange platform needs to be built. As the underlying platform of the application system, it provides a unified integration framework for drug traceability, and provides data processing and delivery services between various business systems and vendors in the supply chain.
2.3 Data Conversion Service
Data conversion services mainly include data extraction, conversion, cleaning and loading services [2]. Data extraction completes data synchronization and migration between multiple types of databases; implements drug data conversion, data cleaning from different vendors, utilizes conditional filtering, removes duplicate records, controls processing, and removes invalid data, and uses data in batch loading form The fastest way to load into the database.
2.4 Data Query Service
The platform provides a unified drug information inquiry service for public users. Through this service, public users can obtain the production and circulation information of drugs in real time. At the same time, the platform can provide the drug supervision and management department with inquiry services to meet the needs of the department. Users can flexibly select the query conditions, and the system can generate corresponding statistical reports according to the user's choice.
3 Application system construction
3.1 Label Distribution Center Construction
The label issuing center completes the issuance management operation of the drug identification label, including label storage management, inventory inquiry, label issuance management, bad label management, label issuance management, label issuance query statistics, label distribution content management, supervision center data synchronization and log, Support functions such as permissions and devices [3].
3.2 Pharmaceutical plant application subsystem construction
The function of the application subsystem of the pharmaceutical factory is mainly divided into the pharmaceutical factory information center, the pharmaceutical factory production line management, the pharmaceutical factory warehouse management, and the supporting function.
The pharmaceutical factory information center is mainly responsible for the collection of drug production information, statistical inquiry, and unified management of drug inventory information, and it is necessary to regularly report the production and inventory information of the drug to the national traceability platform data center.
The production line management system of the pharmaceutical factory needs to renovate the production line, install equipment such as labeling machines, and label the labeling, distribution and management of bad labels.
The pharmaceutical warehouse management system carries out the management of medicines and storage.
3.3 Construction of Imported Drug Management Subsystem
The management of imported drugs completes the customs clearance of imported drugs, the source of drugs, production information, and tracking and management of domestic circulation information. The system mainly includes the import warehouse information center, drug label placement management, import warehouse management and necessary system support functions.
The Import Warehouse Information Center is mainly responsible for the collection of drug import information, statistical inquiry and unified management of imported drug inventory information, and the information of imported drugs should be regularly reported to the National Traceability Platform Data Center.
The drug label placement management system requires the labeling, distribution and collection of bad labels for the packaging of imported medicines.
The imported drug warehouse management system manages the shipment and storage of drugs.
3.4 Wholesale warehousing management subsystem construction
Wholesale warehousing management mainly completes the tracking management of drug information entering the wholesale sales channel, including inventory management, data management and system support functions, and regularly reports the drug's inbound and outbound information and wholesale information to the national traceability platform data center.
3.5 Drug in transit management subsystem construction
The drug in-transit management subsystem uses GPS positioning technology, sensing technology, RFID and other technologies to complete the on-the-go management of drugs [4].
The system reports the location information of the drug, the label information of the drug, and the internal temperature information of the drug carrier to the in-transit management center system in real time through the GPS and RFID readers. The central system saves the in-transit information of the drug and reports the information to the national traceability platform data center.
3.6 Retail Management Subsystem Construction
The retail management subsystem integrates the RFID reader into the drug cash register system [5], completes the real-time collection and management of drug sales information and inbound and outbound information, and reports the information to the national traceability platform data center.
4 Conclusion
Based on the construction of drug traceability management system based on Internet of Things, it proposes to use the advanced Internet of Things technology such as GPS, RFID and temperature sensor to solve the life cycle management problem of drug information, improve the production management level of pharmaceutical enterprises and strengthen the information on imported drugs. Resource management, and comprehensive management of drugs in the process, and effectively do not have a dead end in the whole process, thus curbing counterfeit products, to a certain extent, to solve the problem of drug safety management.
ZGAR PCC KIT
ZGAR electronic cigarette uses high-tech R&D, food grade disposable pod device and high-quality raw material. All package designs are Original IP. Our designer team is from Hong Kong. We have very high requirements for product quality, flavors taste and packaging design. The E-liquid is imported, materials are food grade, and assembly plant is medical-grade dust-free workshops.
From production to packaging, the whole system of tracking, efficient and orderly process, achieving daily efficient output. We pay attention to the details of each process control. The first class dust-free production workshop has passed the GMP food and drug production standard certification, ensuring quality and safety. We choose the products with a traceability system, which can not only effectively track and trace all kinds of data, but also ensure good product quality.
We offer best price, high quality Vape Device, E-Cigarette Vape Pen, Disposable Device Vape,Vape Pen Atomizer, Electronic cigarette to all over the world.
Much Better Vaping Experience!

ZGAR PCC KIT E-Cigarette Vape Pen,ZGAR PCC KIT Disposable Device Vape,PCC SET,ZGAR PCC KIT Vape Pen Atomizer,ZGAR PCC KIT Disposable E-Cigarette OEM vape pen,ZGAR PCC KIT electronic cigarette
Zgar International (M) SDN BHD , https://www.zgarvapepen.com