In order to ensure the stability and reliability of medical devices, the State Council of the People's Republic of China Order No. 276 announced that medical device production must meet strict production conditions. Major medical equipment manufacturers have followed a national regulation and have developed a complete set of production, testing, and after-sales processes to ensure the quality of medical devices.
In the eyes of people, medical devices are more "mysterious", accompanied by "sterile", "precision", "expensive" and other impressions. Along with the rapid development of industrial automation, the production mode of medical devices is also facing innovative reforms. In order to plan stable and efficient production solutions, Zhiyuan Electronics has cooperated with a well-known medical and health electronics company in Tianjin to complete the innovation of new production processes.
After investigation, it is found that most of the medical device manufacturers' equipment production and after-sales are in accordance with the traditional process, using firmware burning, patching, functional testing procedures for production, the process shown in Figure 1.
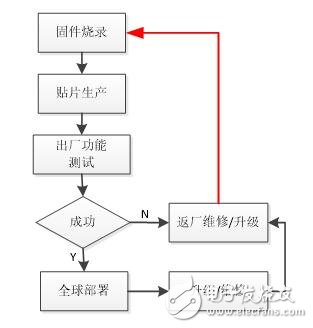
Figure 1 Traditional production and after-sales process of medical devices
In the traditional process, the product function test and the upgrade and maintenance of the equipment need to return the equipment to the factory for maintenance inspection. For medium and large equipment, the maintenance cost of the product will be greatly increased. If it is an urgent customer (such as a hospital, etc.) The upgrade and maintenance will directly affect the normal work, which will inevitably bring great inconvenience.
In order to solve the problem of strong regional requirements for returning to repair and functional testing, ZLG Zhiyuan Electronics has worked closely with a well-known medical and health electronics company in Tianjin to propose a practical and feasible improvement plan to transform the traditional production and maintenance processes. The process after the transformation is shown in Figure 2:
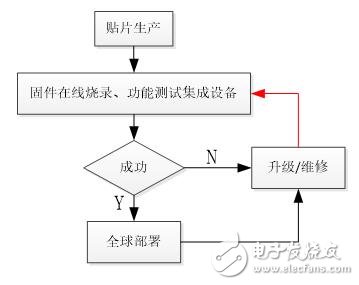
Figure 2 Improvements to the traditional process
The improved process integrates firmware production and functional testing into one, and plans a new type of production test equipment to produce, upgrade, repair and test equipment anytime, anywhere. Users only need to deploy the device to each site that needs to be covered to solve the problems of upgrade and maintenance. This process is more convenient and more efficient, and it will reduce the problem of end users affecting normal use due to fault rework.
In Figure 2, the disruptive innovation lies in the "firmware online burning, functional testing integrated equipment" link, which uses the intelligent programming solution of Zhiyuan Electronics - P800isp mass production online programmer. Mainly on-line programming of the firmware of the product, and real-time testing of the product with the functional test component related to the medical device, can more easily and quickly find and solve the problem.
The P800isp has a rich interface to meet the needs of various remote connections, as well as powerful automation control and intelligent management functions. This is why it is valued by integrated devices.
Remote connection functionP800isp is equipped with interfaces such as network control and a complete API call interface, which can perfectly realize equipment and system integration.

In the medical equipment production line, the P800isp's automatic power-on and power-off detection function is used to automatically perform online programming operations after detecting that the production equipment is in stable contact, and automatically scan the one-dimensional surface of the production equipment using the barcode gun in the integrated equipment during programming. The code tag automatically burns the SN and MAC address of the device to the corresponding area of ​​the chip, which reduces the production control process and greatly reduces the production error rate.

The medical device production details need to be recorded in the form of a report to the server for maintenance, inspection, and auditing. After the programming is completed, the P800isp will provide the programming log to the server for storage in the form of a report, which is convenient for later tracking. P800isp will also give the programming yield rate, which is convenient for the producer to consult, easy to locate and solve the bottleneck of the production process, and find the production failure point.
Cross-regional functionSince medical devices are used around the world and need to be deployed across geographies, in order to solve the upgrade and maintenance of medical device products, programmers equipped with network communication interfaces can be used, and medical device manufacturers can remotely upgrade and upgrade firmware upgrades of devices. Through the remote operation control of the network, it is very convenient for the headquarters to communicate with the after-sales service department to deal with the problem.

The era of Industry 4.0 has arrived, and "Made in China" is also rapidly changing to "China's wisdom." How to quickly improve production efficiency is a difficult problem for major companies. ZLG Zhiyuan Electronics has been committed to providing users with the most reliable programming solutions to help "China's wisdom"!
USB Power Sockets, USB Charger, USB Adaptor, Charging USB Ports, USB Quick Charger
NINGBO COWELL ELECTRONICS & TECHNOLOGY CO., LTD , https://www.cowellsockets.com